Abstract
Introduction: Elotuzumab (Elo) is an immunostimulatory (via natural killer [NK] cell activation) monoclonal antibody (mAb) targeting signaling lymphocytic activation molecule F7 (SLAMF7) that has shown activity in combination with the immunomodulatory drugs (IMiDs) lenalidomide (Len) or pomalidomide (Pom) and dexamethasone (Dex), in patients (pts) with RRMM in the ELOQUENT 2 and 3 trials, respectively. However, in ELOQUENT 2, pts were not Len refractory or previously treated with daratumumab (Dara) and in ELOQUENT 3, <5% of patients were previously treated with Dara. The efficacy of Elo-based regimens in Dara-refractory pts needs prospective evaluation to understand optimal sequencing of regimens in RRMM. We report data from a phase 2 trial evaluating the efficacy of Elo-Pom-Dex (EPd) in pts with Dara-refractory RRMM.
Methods: This phase 2 trial (NCT03713294) enrolled pts with RRMM who had disease progression and were refractory to Dara. Key eligibility criteria included: measurable disease as per IMWG criteria, at least one prior line of therapy, adequate marrow/organ function (neutrophil count ≥1.0 x 109, platelets ≥50x 109, creatinine clearance ≥30 mL/min), ECOG performance status ≤2, and disease not refractory to Pom. EPd was dosed as per the ELOQUENT 3 study. The trial utilized a two-stage design with an interim analysis to test the null hypothesis that the true overall response rate (ORR) in this patient population is most 20% vs. the alternative hypothesis that it is at least 40%, with 90% power and 9% type I error. Treatment was given until disease progression, intolerance, or patient refusal.
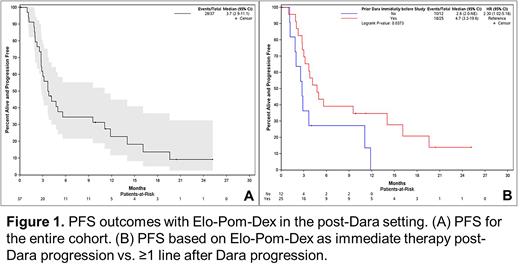
Results: 37 pts were enrolled. Median follow-up time was 23.4 months (95% CI: 14.6-37.2). The median age was 70 years (range: 42-84), 12 (32.4%) pts had ISS III disease and 16 (43.2%) pts had high risk cytogenetics. Pts had received a median of 4 (range: 1-9) prior lines of therapy. Prior anti-MM agents included bortezomib (35; 94.6%), Len (34; 91.9%), carfilzomib (9; 24.3%), ixazomib (9; 24.3%), stem cell transplantation (18; 48.6%) and CAR-T cell therapy (1; 2.7%). The majority of pts (33; 89%) were previously exposed to both a proteasome inhibitor and Len. 25 (67.6%) pts got Dara-based therapy as the immediate line of therapy prior to EPd (after a median of 4 prior lines [range 1-7]) whereas 12 (32%) pts got Dara-based therapy ≥1 line prior to EPd (after a median of 6 prior lines [range 2-9]). ORR in all pts was 35.1% (13 pts), with no CRs, 1 (2.7%) VGPR, and 12 (32.4%) PRs. Four pts (10.8%) achieved an MR and 15 (40.5%) achieved SD. The study met its primary endpoint. The ORR in pts exposed to Len was 11/34 (32.4%) compared to 2/3 (66.7%) in patients not exposed to Len. The ORR in pts with Dara as the immediate prior line of therapy was 44% (11/25) compared to 16.7% (2/12) in pts who had received Dara-based therapy ≥1 line prior to EPd (p=0.15). The median PFS for the entire cohort was 3.7 mth (95% CI: 2.9-11.1) (Figure 1A). The median time to best response was 2.9 mth. The median time to next treatment was 8.5 mth (95% CI: 6.8-20.4). Median PFS of pts with Dara as the immediate prior line of therapy was 4.7 mth (95% CI: 3.2-19.6) compared to 2.8 mth (95% CI: 2.0-NA) in patients who had received Dara-based therapy ≥1 line prior to EPd (p=0.03; Figure 1B). The most common grade 3/4 adverse events were neutropenia (57%), anemia (22%), thrombocytopenia (16%), leucopenia (16%), and fatigue (8%).
Conclusion: This was a positive study with EPd showing clinically meaningful benefit in pts with Dara-refractory RRMM. The reported PFS of 3.7 mths is less than the 10.3 mths reported in ELOQUENT 3 trial which suggests that Elo-based regimens are less effective in Dara-refractory pts. Interestingly, pts had a significantly superior PFS if they received EPd immediately following progression on Dara, however they were less heavily pretreated comparted to pts who got EPd ≥1 line after Dara failure. Nonetheless, EPd is an effective regimen in the post-Dara setting.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal